Some facts
about us
OBERON GmbH Fiber Technologies is one of global leading manufacturers of laser surgery fibers as well as special optic components for medical devices and produces exclusively in Germany. As a certified medical device manufacturer our products are distributed nearly all over the world and we continuously extend our global sales structure.
OBERON Fiber Technologies focuses on the two application areas in photonics: medicine & life sciences and industry & science. We develop and produce special fiber optic components for such areas as laser surgery medicine, medical diagnostics as well as for optical measurement technology in the area of sensory technology and analytics.
Our Compliance is the key to your success
We demand the highest quality of ourselves, and our clients demand it of us. That is why our quality management system conforms to ISO 13485 and ISO 9001 requirements. As a certified medical device manufacturer, OBERON holds all needed certification for the design, development and distribution of medical devices. The conformity of our products has been confirmed by our notified body, so that we can provide devices with ce marking.
Our foundation
Reliability, honesty and diligence, and the ability to react quickly and in an uncomplicated manner, all of this with enthusiasm and passion – that is what drives us. Our customers can rely on our competent and convenient service.
We know the markets and the products, and we feel we know what our clients need and demand when it comes to service, products and technology. We listen to our customers, and we don’t promise anything that we can’t deliver!
We commit ourselves to precision, reliability and customer satisfaction
experience
20 Years
shipped
units
in
2023
315k
customers worldwide
182
production area
>1600 m²
modular ISO class-7 cleanroom
102 m²
design and production exclusively in
Wildau, Berlin
Germany
assembling operators
34
state of the art in production equipment and technology
Since the strategic spin-off from LEONI Fiber Optics in 2013 we perfectly combine over 15 years experience in the manufacturing of optical fibers with the flexibility of a highly specializd, local SME. Over the last 9 years there has been implemented a comprehensive quality management system, which allows us to offer our customers not only products but a full range of services.
key certifications
common solutions and mutual benefits
- long experience combined withcurrent knowledge
- practical solutions for a role as subcontractor
- assistance in preparing relevant documentation
- timely support with local registrations
- recognized validation documents
- Audit support – both from the client or their certifier
We transform customer needs
into precise solutions
Together with a strong network of scientific partners and universities we continiously push developments and always try to find innovative solutions for our customers needs. In various project OBERON successfully developed fibers which allow clinical specialist to enter new fields of application leading to better regeneration of patients, less pain and improved effectiveness of treatments.
InCone
Purpose: Development of a process for laser structuring of distal ends as well as caps for extended aplication areas in minimally invasive medicine.
UroMed
Purpose: Raman-spectroscopy based Diagnostic Fiber Probe in Urology.
Research & Development
Projects in cooperation with:
Statements
Ilja Schenker: Gründer und CEO
„Vertrauen und ein skalierbares Qualitätsmanagement sind die Grundlage unseres Erfolges. Wir haben ein starkes Team und ich sehe es als meine Aufgabe, meinen Kollegen die Rahmenbedingungen für ihr tägliches Wirken zu schaffen.“
Tobias Roth: CEO und verantwortlich für Sales, Marketing, Product Engineering und Controlling
„Kundenorientierung ist für mich keine Worthülse, sondern ein Commitment. Unsere entscheidenden Stärken sind Verlässlichkeit, Reaktivität und Flexibilität.“
Florian Schulz: Quality Management und Regulatory Affairs
„Weltweite regulatorische Anforderungen sind gerade für mittelständische Betriebe eine Herausforderung. Deren Einhaltung ist unser entscheidender Erfolgsfaktor.“
Our foundation:
People, experience, technology and capital
Reliability, honesty and diligence, and the ability to react quickly and in an uncomplicated manner, all of this with enthusiasm and passion – that is what we seek as a team, and that is what drives us. Our customers can rely on our competent and convenient service.
We know the markets and the products, and we feel we know what our clients need and demand when it comes to service, products and technology. We listen to our customers, and we don’t promise anything that we can’t deliver!
OBERON Fiber Technologies focuses on the two application areas in photonics: medicine & life sciences and industry & science. We develop and produce special fiber optic components for such areas as laser surgery medicine, medical diagnostics as well as for optical measurement technology in the area of sensory technology and analytics.
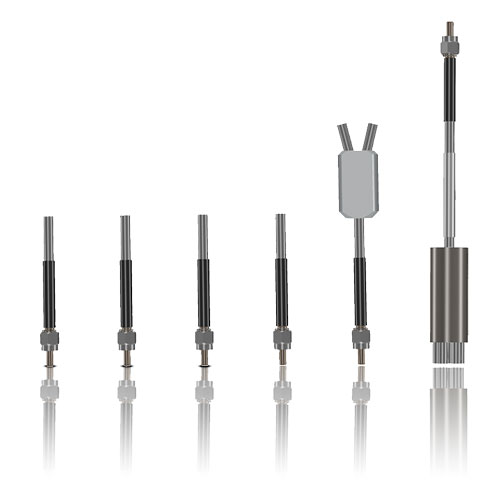
Our experience is your advantage
The basis of optical laser and measurement systems is the transmission of photons using our product, special system components with internal silica fibers. Our product portfolio includes fiber optical probes and special assemblies for transmitting visible and invisible light. In development and manufacturing, we distinguish between two technological applications: power transmission (laser beam delivery) and measurement data logging and transmission. One example of this kind of application is spectroscopy.
OBERON Fiber Technologies is a company with an experienced team. Every one of our company’s employees, whether in production, quality control, sales or product design, has over ten years of experience in his or her field.
OBERON Fiber Technologies operates with a minimum level of hierarchy, a minimum level of overhead and a maximum level of cost savings, which we pass on to our clients.
Triple safety standards for our product
OBERON Fiber Technologies takes quality seriously – quality that forms the basis for safety. Safety in the process, safety for the user, safety for the patient.
We demand reproducible quality of ourselves, and our clients demand it of us. That is why our quality management system conforms to EN ISO 9001 requirements.
OBERON Fiber Technologies meets the prerequisites to manufacture medical devices and bring them to market. We have carried out a conformity evaluation procedure in accordance with Annex II, Article 3 of Directive 93/42/EWG and implemented a comprehensive quality control system. As a result, we are licensed to give our medical devices a CE marking. We have initiated the supplemental quality management system in compliance with EN ISO 13485 requirements.
Medical product safety
OBERON Fiber Technologies has its medical Laser Surgery Fiber licensed with the USFDA under 510k no. K140470.
OBERON Fiber Technologies has sterility certifications in accordance with EN ISO 11135-1, endotoxin-free certifications, EO and ECH residual analyses and Bioburden tests for the medical devices we manufacture. The requirements under EN ISO 10993-1 on biocompatibility are met. The EtO sterility process is validated. All materials used for the packaging of our medical devices meet DIN EN ISO 11607 and DIN EN 868 requirements. We achieve reproducible environmental conditions in our ISO class-7 clean room. Clean room monitoring is regularly carried out by an accredited laboratory.